Dracunculiasis
| Dracunculiasis | |
|---|---|
| Classification and external resources | |
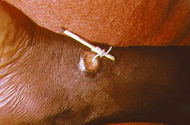 A method used to extract a guinea worm from the leg vein of a human. |
|
| ICD-10 | B72. |
| ICD-9 | 125.7 |
| DiseasesDB | 3945 |
| eMedicine | ped/616 |
| MeSH | D004320 |
Dracunculiasis, also called guinea worm disease (GWD), is a parasitic infection caused by Dracunculus medinensis, a long and very thin nematode (roundworm).[1] The infection begins when a person drinks stagnant water contaminated with microscopic "water fleas" harboring the larvae of the guinea worm. Approximately one year later, the disease presents with a painful, burning sensation as the worm forms a blister, usually on the lower limb. Once prevalent in 20 nations in Asia and Africa, the disease remains endemic in only four countries in Africa.
The guinea worm is one of the best historically documented human parasites, with tales of its behaviour reaching as far back as the second century BC in accounts penned by Greek chroniclers.[2] The name dracunculiasis is derived from the Latin "affliction with little dragons"[3] while the common name "guinea worm" appeared after Europeans saw the disease on the Guinea coast of West Africa in the 17th century.[4]
The Carter Center has predicted that guinea worm disease will be the first parasitic disease to be eradicated and the first disease to be eradicated through behavior change, without the use of vaccines or a cure.[5] There is no animal or environmental reservoir of D. medinensis and thus the parasite must pass through humans each year to survive.[5]
Contents |
Signs and symptoms

As the worm moves downwards, usually to the lower leg, through the subcutaneous tissues it leads to intense pain localized to its path of travel. The painful, burning sensation experienced by infected people has led to the disease being called "the fiery serpent". Other symptoms include: fever, nausea, and vomiting.[6]
Cause
Guinea worm disease is caused by drinking water contaminated by water fleas (copepods) containing the Dracunculus larvae.[6]
Life cycle
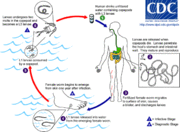
Guinea worm disease used to thrive in some of the world's poorest areas, particularly those with limited or no access to clean water.[7] In these areas stagnant water sources may still host microscopic, fresh-water arthropods known as copepods, which can carry the larvae of the guinea worm.
The larvae develop for approximately two weeks inside the copepods. At this stage the larvae can cause guinea worm disease if the infected copepods are not filtered from drinking water.[4] The male guinea worm is typically much smaller (12–29 mm or 0.47–1.1 in) than the female, which, as an adult, can grow to 2–3 feet (0.61–0.91 m) long and be as thick as a spaghetti noodle.[4][7]
Once inside the body, stomach acid digests the water flea, but not the guinea worm larvae that is sheltered inside. These larvae find their way to the body cavity where the female mates with a male guinea worm. This takes place approximately three months after infection. The male worm dies after mating and is absorbed.[4]
The female, which contains larvae, burrows into the deeper connective tissues or adjacent to long bones or joints of the extremities.[4]
Approximately one year after the infection began, the worm attempts to leave the body by creating a blister in the human host's skin—usually on a person's lower extremities like a leg or foot.[8] Within 72 hours the blister ruptures, exposing one end of the emergent worm. This blister causes a very painful burning sensation as the worm emerges. Infected persons often immerse the affected limb in water to relieve the burning sensation. Once the blister or open sore is submerged in water, the adult female releases hundreds of thousands of guinea worm larvae, contaminating the water supply.
During the next few days, the female worm is capable of releasing more larvae whenever it comes in contact with water. These larvae contaminate the water supply and are eaten by copepods, thereby repeating the life-cycle of the disease. Infected copepods can only live in the water for two to three weeks if they are not ingested by a person. Infection does not create immunity, so people can repeatedly experience guinea worm disease throughout their lives.[7]
In drier areas just below the Sahara desert, cases of the disease often emerge during the rainy seasons, which for many agricultural communities is also the planting or harvesting season. Elsewhere the emerging worms are more prevalent during the dry season, when scarce surface water is most polluted. Guinea worm disease outbreaks can cause serious disruption to local food supplies and school attendance.[7]
Prevention

Guinea worm disease can only be transmitted by drinking contaminated water, and can be completely prevented through relatively simple measures that could result in the disease being eradicated:[6]
- Drinking solely water drawn from underground sources free from contamination, such as a borehole or hand-dug wells.
- Filtering drinking water, using a fine-mesh cloth filter like nylon, to remove the guinea worm-containing crustaceans.
- Preventing people with emerging guinea worms from entering ponds and wells used for drinking water.
- Developing new sources of drinking water that lack the parasites, or repairing dysfunctional ones.
Water sources can also be treated with larvicides to kill worm-carrying crustaceans.[9]
Treatment
There is no vaccine or medicine to treat or prevent Guinea worm disease.[6] Once a Guinea worm begins emerging, a person must wrap the live worm around a piece of gauze or a stick to extract it from the body (a long, painful process which usually takes weeks or months).[5] This is the same treatment that is noted in the famous ancient Egyptian medical text, the Ebers papyrus from 1550 B.C.[4] Some people have said that extracting a Guinea worm feels like the afflicted area is on fire.[10][11] However, if the infection is identified before an ulcer forms, the worm can also be surgically removed by a trained doctor in a medical facility.[7]
Although Guinea worm disease is usually not fatal, the wound where the worm emerges could develop a secondary bacterial infection such as tetanus, which may be life-threatening—a concern in endemic areas where there is typically limited or no access to health care.[12] Analgesics can be used to help reduce swelling and pain and antibiotic ointments can help prevent secondary infections at the wound site.[7]
Use of metronidazole or thiabendazole may make extraction easier, but also may lead to migration to other parts of the body.[13]
Epidemiology
In 1986, there were an estimated 3.5 million cases of Guinea worm in 20 endemic nations in Asia and Africa.[5] The number of cases has been reduced by more than 99% to 3,190 in 2009, 3,185 of them in the four remaining endemic nations of Africa: Sudan, Ghana, Mali and Ethiopia. This is the lowest number of cases since the eradication campaign began. As of 2010, however, the WHO predicted it will be "a few years yet" before eradication is achieved, on the basis that it took 6–12 years for the countries that have so far eliminated Guinea worm transmission to do so after reporting a similar number of cases to that reported by Sudan in 2009.[14] In the first six months of 2010, the number of cases has been reduced by a further 47% compared to the same period in 2009, from 1,441 to 764. All four remaining endemic countries succeeded in reducing their case totals further during this period, with Ghana in particular achieving a 96% reduction from 228 cases in January-June 2009 to 8 cases in January-June 2010, all of them contained.[15]
The World Health Organization is the international body that certifies whether a disease has been eliminated from a country or eradicated from the world.[16] The Carter Center also reports the status of the Guinea worm eradication program by country.[17]
Certified free
Endemic countries must report to the International Commission for the Certification of Dracunculiasis Eradication and document the absence of indigenous cases of Guinea worm disease for at least three consecutive years to be certified as Guinea worm-free by the World Health Organization.[18]
The results of this certification scheme have been remarkable: by 2007, Benin, Burkina Faso, Chad, Côte d'Ivoire, Kenya, Mauritania, Togo, and Uganda had stopped transmission, and Cameroon, CAR, India, Pakistan, Senegal, Yemen were WHO certified.[17]
Endemic
Sudan, Ghana, Mali and Ethiopia still have endemic transmission as of early 2010. The major focus is now southern Sudan, which reported 86% of all cases in 2009. [19][14]
| Country | Location | 2007 | 2008 | 2009 | Description |
|---|---|---|---|---|---|
| Sudan |  |
5,815[20] | 3,618[20] | 2,733[14] | Sudan has greatly ratcheted up its eradication effort since 2005 when the Comprehensive Peace Agreement was signed, ending a more than two-decade long civil war. 2006 saw an increase to 15,539 cases of Guinea Worm disease, from 5,569 cases in 2005, as a result of reporting from endemic areas that were no longer war-torn. The Southern Sudan Guinea Worm Eradication Program (SSGWEP) has deployed over 28,000 village volunteers, supervisors and other health staff to work on the program full time. The SSGWEP was able to slash the number of cases reported in 2006 by 63% to 5,815 cases in 2007. Northern Sudan has reported no endemic cases of dracunculiasis since 2001.[21] |
| Ghana |  |
3,358[20] | 501[20] | 242[14] | In Ghana, after a decade of frustration and stagnation, in 2006 a decisive turnaround was achieved. Multiple changes can be attributed to the improved containment and lower incidence of dracunculiasis: better supervision and accountability, active oversight of patients daily by paid staff, and an intensified public awareness campaign. After Jimmy Carter’s visit to Ghana in August 2006, the government of Ghana declared Guinea worm disease to be a public health emergency. The overall rate of contained cases has increased in Ghana from 60% in 2005, to 75% in 2006, 84% in 2007, 85% in 2008 and to 93% in 2009 [19][21] |
| Mali |  |
313[20] | 417[20] | 186[14] | Four of Mali’s regions (Kayes, Koulikoro, Segou, and Sikasso) have eliminated dracunculiasis, while the disease is still endemic in the country’s other four regions (Gao, Kidal, Mopti, and Timbuktu). Late detection of two outbreaks, due to inadequate surveillance, in 2007 resulted in a meager 36% containment rate in Mali in 2007.[21] 2008 and 2009 were more successful, however, with containment rates of 85% and 73% respectively.[19] |
| Ethiopia |  |
0[20] | 41[20] | 24[14] | Prior to March 2008, there had been no cases reported in Ethiopia since June 2006.[22] |
Attempting eradication
The global campaign to eradicate Guinea worm disease began at the U.S. Centers for Disease Control and Prevention (CDC) in 1980. In 1986, former U.S. President Jimmy Carter and his not-for-profit organization, The Carter Center, began leading the global campaign, in conjunction with CDC, UNICEF, and WHO.[23] At this time India, Pakistan, Yemen and 17 countries in Africa were endemic for this disease and reported a total of 3.5 million cases per year.
Carter made a personal visit to a Guinea worm endemic village in 1988 he said: "Encountering those victims first-hand, particularly the teenagers and small children, propelled me and Rosalynn to step up the Carter Center's efforts to eradicate Guinea worm disease."[24]
President Carter also recruited two African former heads of state to the battle against Guinea worm disease. Then-former head of state of Mali, General Amadou Toumani Toure (since elected President of Mali) has been a strong advocate of Guinea worm eradication in Mali and all other French-speaking African endemic countries since 1992.[25][26] Since 1999, former Nigerian head of state General (Dr.) Yakubu Gowan has played a similar role in Nigeria, which at the eradication campaign's start had more cases than any other country.[27]
Since humans are the only host for Guinea worm, the disease can be controlled by identifying all cases and modifying human behavior to prevent it from recurring.[5] Once all human cases are eliminated, the disease cycle will be broken, resulting in its eradication.[5]
In 1991, the World Health Assembly (WHA) agreed that Guinea worm disease should be eradicated.[12] At this time there were 400,000 cases reported each year. The Carter Center has continued to lead the eradication efforts, primarily through its Guinea Worm Eradication Program.[28] Other major actors in the eradication of Guinea worm disease include: World Health Organization, U.S. Centers for Disease Control and Prevention, Bill & Melinda Gates Foundation, and UNICEF,[28][29] but the global coalition now includes dozens of other donors, nongovernmental organizations, and institutions, most especially the ministries of health of the affected countries themselves.
In December 2008, The Carter Center announced new financial support totaling US $55 million from the Bill & Melinda Gates Foundation and the United Kingdom Department for International Development.[30] The funds will help address the higher cost of identifying and reporting the last cases of Guinea worm disease. According to The Carter Center, surveillance of countries, including the smallest communities in the most remote areas, needs to be intensified to prevent outbreaks and setbacks. In the case of Guinea worm disease, which has a one-year incubation period, there is a very high cost of maintaining a broad and sensitive monitoring system and providing a rapid response when necessary.[30]
Barriers
The eradication of Guinea worm disease has faced several challenges:
-
- inadequate security in some endemic countries;
- lack of political will from the leaders of some of the countries in which the disease is endemic;
- the need for change in behavior in the absence of a ‘magic bullet’ treatment like a vaccine or medication; and
- inadequate funding at certain times.[3]
One of the most significant challenges facing Guinea worm eradication has been the civil war in southern Sudan, which was largely inaccessible to health workers due to violence.[3][31] To address some of the humanitarian needs in southern Sudan, in 1995, the longest ceasefire in the history of the war was achieved through negotiations by Jimmy Carter.[3][31] Commonly called the "Guinea worm cease-fire," both warring parties agreed to halt hostilities for nearly six months to allow public health officials to begin Guinea worm eradication programming, among other interventions.[31][32]
Public health officials cite the formal end of the war in 2005, as a turning point in Guinea worm eradication because it has allowed health care workers greater access to southern Sudan's endemic areas.[29] One remaining area in West Africa outside of Ghana remains challenging to ending Guinea worm: northern Mali, where Tuareg rebels have made some affected areas unsafe for health workers.
One of the greater challenges ahead in eradicating dracunculiasis will be confronted in Sudan: there is much uncertainty with future political benchmarks in Sudan (national elections in 2009 and the referendum on the status of southern Sudan in 2011). Sporadic insecurity or widespread civil conflict could at any time ignite and thwart eradication efforts.[21] The remaining endemic communities in Sudan are remote, poor and devoid of infrastructure, presenting significant hurdles for effective delivery of interventions against disease. Moreover, residents in these communities are nomadic, moving seasonally with cattle in pursuit of water and pasture, making it very difficult to know where and when transmission occurred. The peak transmission season coincides with the rainy season, hampering travel by public health workers.[33]
Society and culture
The pain caused by the worm's emergence—which typically occurs during planting and harvesting seasons—prevents many people from working or attending school for as long as three months. In heavily burdened agricultural villages fewer people are able to tend their fields or livestock, resulting in food shortages and lower earnings.[5][29] A study in southeastern Nigeria, for example, found that rice farmers in a small area lost US$20 million in just one year due to outbreaks of Guinea worm disease.[5]
History
Dracunculiasis has been a recognized disease for thousands of years:
- Guinea worm may be the "fiery serpent" that plagued the Israelites in the Old Testament: "And the Lord sent fiery serpents among the people, and they bit the people; and much people of Israel died." (Numbers 21:4-9).[4]
- The 2nd century B.C., Greek writer Agatharchides, described this affliction as being endemic amongst certain nomads in what is now Sudan and along the Red Sea.[4]
- The traditional (and still current) method of extracting guinea worm by twisting the worm around a stick may have inspired the rod of Asclepius, a symbol of medicine since Ancient Greek times which portrays a snake winding around a staff, and which is often confused with the Caduceus.[34]
- The unusually high incidence of dracunculiasis in the city of Medina led to it being included in part of the disease's scientific name "medinensis." A similar high incidence along the Guinea coast of West Africa gave the disease its more commonly used name.[4] Guinea worm is no longer endemic in either location.
In modern times, the first who described dracunculiasis and its pathogenesis was the Bulgarian physician Hristo Stambolski, during his exile in Yemen (1877–1878).[35] His theory was that the cause was infected water which people were drinking.
See also
- Infectious disease in the 20th century
- List of infectious diseases
- List of parasites (human)
- Tropical disease
- Neglected disease
- Waterborne diseases
- Eradication of infectious disease
References
- ↑ James, WD; Berger TG et al. (2006). Andrews' Diseases of the Skin: Clinical Dermatology. Saunders Elsevier. pp. 437. ISBN 0-7216-2921-0.
- ↑ Piper R (2007). Extraordinary Animals: An Encyclopedia of Curious and Unusual Animals. Greenwood Press. pp. 189-91. ISBN 978-0313339226.
- ↑ 3.0 3.1 3.2 3.3 Barry, M (2007-06-21). "The Tail End of Guinea Worm — Global Eradication without a Drug or a Vaccine". New England Journal of Medicine 356 (25): 2561–2564. doi:10.1056/NEJMp078089. PMID 17582064. http://content.nejm.org/cgi/content/full/356/25/2561. Retrieved 2008-07-15.
- ↑ 4.0 4.1 4.2 4.3 4.4 4.5 4.6 4.7 4.8 Tropical Medicine Central Resource. "Dracunculiasis". Uniformed Services University of the Health Sciences. http://www.isradiology.org/tropical_deseases/tmcr/chapter27/intro.htm. Retrieved 2008-07-15.
- ↑ 5.0 5.1 5.2 5.3 5.4 5.5 5.6 5.7 5.8 "Guinea Worm Eradication Program". The Carter Center. Carter Center. http://www.cartercenter.org/health/guinea_worm/index.html. Retrieved 2008-07-15.
- ↑ 6.0 6.1 6.2 6.3 "WHO | Dracunculiasis". World Health Organization. http://www.who.int/topics/dracunculiasis/en/. Retrieved 2010-07-12.
- ↑ 7.0 7.1 7.2 7.3 7.4 7.5 "Fact Sheet:Dracunculiasis - Guinea Worm Disease". CDC. 2008-07-15. http://www.cdc.gov/ncidod/dpd/parasites/dracunculiasis/factsht_dracunculiasis.htm. Retrieved 2010-07-12.
- ↑ CDC (2007-08-15). "Progress Toward Global Eradication of Dracunculiasis January 2005 — May 2007". Mortality and Morbidity Weekly Report 56 (32): 813–817. http://www.cdc.gov/mmwr/preview/mmwrhtml/mm5632a1.htm. Retrieved 2010-07-12.
- ↑ PMID 17954680 (PubMed)
Citation will be completed automatically in a few minutes. Jump the queue or expand by hand - ↑ "World moves closer to eradicating ancient worm disease". =World Health Organization. 2007-03-27. http://www.who.int/mediacentre/news/notes/2007/np15/en/index.html. Retrieved 2008-07-15.
- ↑ McNeil, DG (2006-03-26). "Dose of Tenacity Wears Down a Horrific Disease". The New York Times. http://www.nytimes.com/2006/03/26/international/africa/26worm.html. Retrieved 2008-07-15.
- ↑ 12.0 12.1 CDC (1993-12-13). "Recommendations of the International Task Force for Disease Eradication". Mortality and Morbidity Weekly Report 42 (RR16): 1–25. http://www.cdc.gov/mmwr/preview/mmwrhtml/00025967.htm. Retrieved 2008-07-15.
- ↑ "Dracunculiasis: Treatment & Medication". eMedicine. http://emedicine.medscape.com/article/997617-treatment. Retrieved 2010-05-02.
- ↑ 14.0 14.1 14.2 14.3 14.4 14.5 World Health Organization. "Dracunculiasis eradication - global surveillance summary, 2009" (pdf). Weekly Epidemiology Record 85 (19): 166. http://www.who.int/entity/wer/2010/wer8519.pdf. Retrieved 2010-05-14.
- ↑ "Guinea Worm Wrap-Up 198, 7 July 2010" (pdf). Carter Center. http://cartercenter.org/resources/pdfs/news/health_publications/guinea_worm/wrap-up/198.pdf. Retrieved 2010-07-12.
- ↑ "WHO certifies seven more countries as free of guinea-worm disease". World Health Organization. http://www.who.int/neglected_diseases/guineaworm_press_note/en/index.html. Retrieved 2010-05-14.
- ↑ 17.0 17.1 "Activities by Country - Guinea Worm Eradication Program". Carter Center. http://www.cartercenter.org/countries/activities_gw.html. Retrieved 2010-03-16.
- ↑ CDC (October 11, 2000). "Progress Toward Global Dracunculiasis Eradication, June 2000". Mortality and Morbidity Weekly Report 49 (14): 731–735. doi:10.1001/jama.284.14.1778. PMID 11041744. http://jama.ama-assn.org/cgi/content/full/284/14/1778. Retrieved July 15, 2008.
- ↑ 19.0 19.1 19.2 "Guinea worm wrap-up 195, 12 March 2010" (pdf). Carter Center. http://cartercenter.org/resources/pdfs/news/health_publications/guinea_worm/wrap-up/195.pdf. Retrieved 2010-03-16.
- ↑ 20.0 20.1 20.2 20.3 20.4 20.5 20.6 20.7 "Progress toward Global Eradication of Dracunculiasis, January 2008–June 2009". Medscape. http://www.medscape.com/viewarticle/711296. Retrieved 2010-05-02.
- ↑ 21.0 21.1 21.2 21.3 Donald R. Hopkins, Ernesto Ruiz-Tiben, Philip Downs, P. Craig Withers, Jr. & Sharon Roy (2008), "Dracunuliasis eradication: neglected no longer", American Journal of Tropical Medicine and Hygiene 79 (4): 474–479, PMID 18840732.
- ↑ "Update: Progress Toward Global Eradication of Dracunculiasis, January 2007--June 2008". http://www.cdc.gov/mmwr/preview/mmwrhtml/mm5743a3.htm. Retrieved 2010-05-02.
- ↑ The Carter Center, "International Task Force for Disease Eradication - Original Members (1989–1992)", http://www.cartercenter.org/health/itfde/original_members.html, retrieved July 17, 2008
- ↑ Carter, Jimmy; Lodge, Michelle (March 31, 2008), "A Village Woman's Legacy" (PDF), TIME, http://www.cartercenter.org/resources/pdfs/news/health_publications/guinea_worm/time_village_womans_legacy_mar31_08.pdf, retrieved July 15, 2008
- ↑ Jimmy Carter (May 19, 2004), "Remarks of Mr Jimmy Carter, former President of the United States of America, at the World Health Assembly", World Health Organization, http://www.who.int/mediacentre/events/2004/wha57/carter/en/, retrieved July 17, 2008
- ↑ The Carter Center, Carter (April 2, 2008), "Mali's President Touré, Southern Sudan Program Director Logora Honored With The Jimmy and Rosalynn Carter Award", http://www.cartercenter.org/news/pr/toure_honored.html, retrieved July 17, 2008
- ↑ The Carter Center (April 2, 2008), "Jimmy Carter and General Dr. Yakubu Gowon Encourage Nigerian Officials to Control Schistosomiasis, Other Diseases", http://www.cartercenter.org/news/pr/nigeria_021507.html, retrieved July 17, 2008
- ↑ 28.0 28.1 The Carter Center (2006), 2006 Gates Award for Global Health: The Carter Center, http://www.cartercenter.org/news/documents/nondatabase/speech_gates.html, retrieved March 8, 2010
- ↑ 29.0 29.1 29.2 Donald R. Hopkins, Ernesto Ruiz-Tiben, Philip Downs, P. Craig Withers, Jr. & James H. Maguire (October 1, 2005), "Dracunculiasis Eradication: The Final Inch", American Journal of Tropical Medicine and Hygiene 73 (4): 669–675, PMID 16222007, http://www.ajtmh.org/cgi/content/abstract/73/4/669, retrieved July 15, 2008
- ↑ 30.0 30.1 The Carter Center, Guinea Worm Cases Hit All-Time Low: Carter Center, WHO, Gates Foundation, and U.K. Government Commit $55 Million Toward Ultimate Eradication Goal, http://www.cartercenter.org/news/pr/gates_120508.html, retrieved December 8, 2008
- ↑ 31.0 31.1 31.2 The Carter Center, Sudan, http://www.cartercenter.org/countries/sudan.html, retrieved July 15, 2008
- ↑ Hopkins, Donald R.; Withers, P. Craig, Jr. (2002), "Sudan's war and eradication of dracunculiasis", The Lancet 360: s21–s22, doi:10.1016/S0140-6736(02)11806-X
- ↑ WHO. Weekly Epidemiological Record. No. 18, 2008, 83, 157–68.
- ↑ Keith Blayney (2002). "Caduceus vs Staff of Asclepius". http://www.drblayney.com/Asclepius.html. Retrieved June 24, 2009.
- ↑ Христо Стамболски: Автобиография, дневници и mспомени. (Autobiography of Hristo Stambolski. Sofia : Dŭržavna pečatnica, 1927–1931)
External links
- The Carter Center Guinea Worm Disease Eradication Program
- Tropical Medicine Central Resource: "Guinea Worm Infection (Dracunculiasis)"
- World Health Organization on Dracunculiasis
- Weekly epidemiological record of WHO - Dracunculiasis eradication - global surveillance summary, 2009
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||